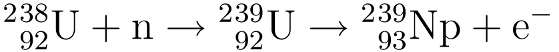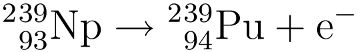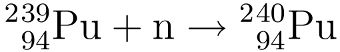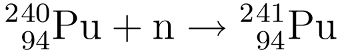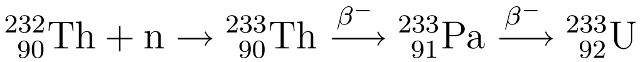Nuclear waste is often quoted as having a “half-life of millions of years” as if this is a bad thing in and of itself.* But there’s another way of looking at it.
Radioactive decay occurs when an unstable atom emits either a helium nucleus, a high-speed electron, an electromagnetic wave called a gamma ray or more rarely one of a number of other possibilities. Being in the way of these emitted particles and waves is generally considered to be a Very Bad Idea.
Radioactive decay occurs at random, with each atom having a chance of decaying at any given moment. The more likely it is that atoms decay, the quicker they decay, and the shorter their half-life.

Imagine the radioactive atoms are ammunition cartridges; when they decay the cartridge “goes off” and a bullet is released. Now imagine you’re standing next to two piles of cartridges representing some nuclear waste: one pile with a short half-life and one pile with a long half-life
The bullets in the short half-life pile will go off over a short period of time, and the bullets in the long half-life pile will go off over a longer period of time. Which pile would be safer to stand next to?
Caesium-135 and caesium-137 are both common isotopes found in nuclear waste: Cs-135 is formed when xenon-135 produced as a fission fragment decays by beta emission; and Cs-137 is formed as a fission fragment itself (a uranium nucleus splits to form one caesium-137 and one rubidium-98 nucleus).
Cs-135 has a half-life of 2.3 million years and emits beta particles with an energy of 267 keV. Cs-137 has a half-life of 30 years and emits beta particles with an energy of 605000 keV. On a graph of 100 years the change in caesium-135 is invisible; only at a scale of a million years does the change become visible:
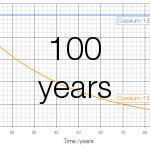
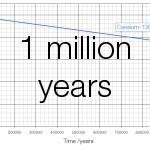
If you stood next to a million atoms of Cs-137 for a year 22840 atoms would decay, for a total energy release of 2.2 nanojoules. Standing next to a million atoms of Cs-135 for a year less than one atom (0.301) would decay and the total energy released would be 13 femtojoules, less than 150 thousandth of the energy released by the caesium-137.
So you have a tradeoff: caesium-135 is less dangerous than caesium-137 but becomes less dangerous more quickly. Both Cs-135 and Cs-137 decay to form stable (non-radioactive) barium so if you can turn a profit selling barium then you’re better off buying a truckload of Cs-137; you’ll be able to sell it as barium sooner.
* It’s worth bearing in mind that nuclear waste eventually becomes safe. Chemical waste from the production of solar cells like silicon tetrafluoride and cadmium telluride remain toxic forever.








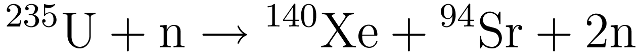
 Part of the SuperKamiokande detector, with Japanese physicists and rubber dingy for scale.
Part of the SuperKamiokande detector, with Japanese physicists and rubber dingy for scale.