It used to be the case that coins used as currency had value because of the material from which they were made: a solid gold coin weighing one ounce had the same value as one ounce of gold. The introduction of fiat currencies, issued by central banks, that only have value because a government decrees by law that they do, allowed governments to move away from this dependence on rare metals.

UK coins come in four “flavours”: the “copper” 1p and 2p coins, the “silver” 5p, 10p, 20p and 50p coins and the larger and heavier £1 and two-tone £2 coins. Before 1992 the “copper” 1p and 2p coins really did contain copper, being made from a bronze containing 97% copper, 2.5% zinc and 0.5% tin. With a mass of 3.56 and 7.12 grams respectively the pre-1992 coins contained 3.54 and 6.91 grams of copper; at the current bulk price and the current USD-GBP exchange rate this makes a 1p coin worth 1.66 pence and a 2p coin worth 3.23 pence (those links will take you to automatically calculated values using current values for spot price and exchange rate).
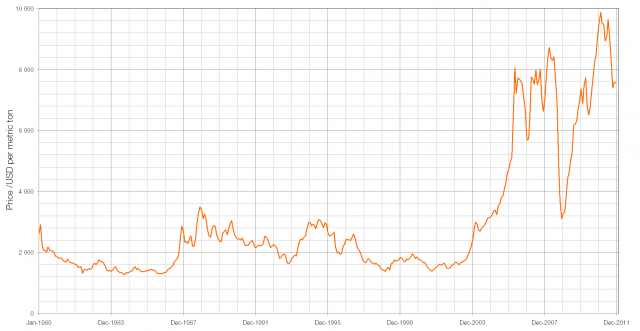 If you were able to buy pre-1992 1p and 2p coins in bulk for their face value you could make a profit by melting them down and selling the resulting copper as scrap (though this is illegal under the 1971 Coinage Act). This increasing price of copper in the early lead to a change from bronze to copper-plated steel and this makes it very easy to differentiate between the old and new 1p/2p coins: pre-1992 coins are not magnetic and post-1992 coins are.
If you were able to buy pre-1992 1p and 2p coins in bulk for their face value you could make a profit by melting them down and selling the resulting copper as scrap (though this is illegal under the 1971 Coinage Act). This increasing price of copper in the early lead to a change from bronze to copper-plated steel and this makes it very easy to differentiate between the old and new 1p/2p coins: pre-1992 coins are not magnetic and post-1992 coins are.
The “silver” coins have previously been made from cupro-nickel, an alloy of copper and nickel in a 75:25 ratio. In terms of its raw metal, a 3.25 gram five pence coin, containing 2.44 grams of copper and 0.81 grams of nickel is currently worth 3.08 pence (1.14p for the copper and 1.94p for the nickel). A 6½ gram ten pence coin is worth 6.16p, a five gram twenty pence coin is worth 4.74p and an eight gram fifty pence coin is worth 7.58p.
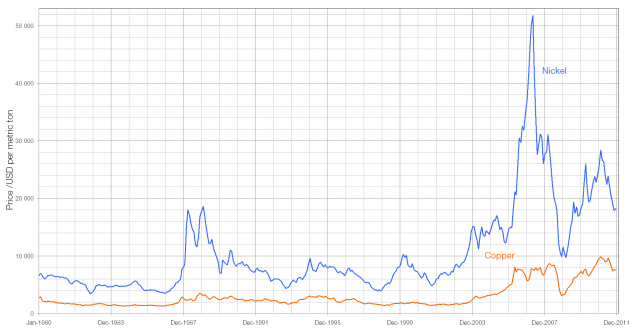
From January 2012 the “silver” coins will be made from nickel-plated steel (making them magnetic), a change driven probably by the rising cost of both copper and nickel. (In May 2007, when the price of nickel was at a peak, a five pence coin was worth more than six pence as scrap copper and nickel).
 The high value of the £1 coin (current value), composed of 70% copper, 24.5% zinc, and 5.5% nickel and the £2 coin, with a cupro-nickel core surrounded by a ring of 76% copper, 20% zinc, and 4% nickel, makes them unlikely to ever approach a situation in which they are worth more than their face value.
The high value of the £1 coin (current value), composed of 70% copper, 24.5% zinc, and 5.5% nickel and the £2 coin, with a cupro-nickel core surrounded by a ring of 76% copper, 20% zinc, and 4% nickel, makes them unlikely to ever approach a situation in which they are worth more than their face value.