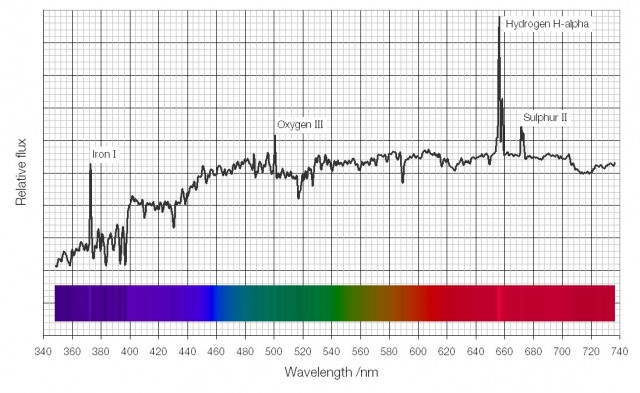
There are many circumstances in which a system will emit light.
During incandescence objects emit light because of their temperature. Everything above absolute zero emits electromagnetic radiation due to its temperature as the electrons in the object vibrate back and forth due to the motion of the atoms that make up the object. The type and amount of EM radiation emitted depends on the temperature – this is how infrared thermometers work, by measuring the intensity and wavelength of the IR radiation emitted.

A red-hot piece of metal demonstrating incandescence.
Luminescence is the emission of light not due to temperature, and can be broken up into many sub-processes, listed below.
Fluorescence is one of the most familiar of these processes. An object fluoresces when it absorbs electromagnetic energy of one sort and subsequently emits another, usually longer-wavelength energy. This is how hidden “ultraviolet ink” works and why clothes sometimes “glow” under UV illumination. Fluorescence is a subset of photoluminescence, in which light emission is the result of absorption of photons, with the other photoluminescent process being phosphorescence, a much slower process than fluorescence in which the emission of photons is highly delayed. It is phosphorescence which is responsible for the light produced by glow-in-the-dark materials that are “charged” by light.
 A selection of fluorescent minerals.
A selection of fluorescent minerals.
 A phosphorescent statue.
A phosphorescent statue.
Chemiluminescence is the process by which light is emitted during a chemical reaction, such as the reaction which occurs in glow sticks. Bioluminescence is a subset of this, when the process occurs in living organisms like fireflies. The other subset of chemiluminescence, electrochemiluminescence occurs when an voltage is applied to a solution; this is how LEDs operate. Cathodoluminescence is itself a subset of electroluminescence, occurring when electrons strike a material such as a phosphor, causing the electron’s energy to be converted to light. This is how old-fashioned cathode ray tube (CRT) televisions operate.
 A solution of luminol demonstrating chemiluminescence.
A solution of luminol demonstrating chemiluminescence.
 L-R: A squid and a firefly demonstrating bioluminescence.
L-R: A squid and a firefly demonstrating bioluminescence.
 A selection of electroluminescent blue LEDs.
A selection of electroluminescent blue LEDs.
Crystalloluminescence is the process by which light is emitted during crystallisation and fractoluminescence when the bonds in crystals are broken.
Fractoluminescence is a subset of mechanoluminescence in which light is emitted as a result of forces acting on a solid. Other mechanoluminescent processes include triboluminescence in which the action of friction causes light to be emitted as chemical bonds in a substance are broken; piezoluminescence in which the action of pressure on a solid causes light to be emitted as electrons and holes recombine; and sonoluminescence is which bubbles in liquids excited by sound waves collapse, emitting light in the process. The exact process that causes sonoluminescence is unknown, though many suggestions including bremsstrahlung radiation, coronal discharge and proton tunnelling have been suggested.
Radioluminescence occurs when light is emitted as the result of bombardment by ionising radiation. It is radioluminescence that was previously used in glow-in-the-dark materials (in particular radium dials) and which is responsible for the glow produced by tritium illumination.
 A radioluminescent tritium light source.
A radioluminescent tritium light source.
Finally, thermoluminescence occurs when certain crystalline materials emit energy they had previously absorbed in the form of EM radiation or via bombardment of ionising radiation as a result of being heated.


 A selection of fluorescent minerals.
A selection of fluorescent minerals. A phosphorescent statue.
A phosphorescent statue. A solution of luminol demonstrating chemiluminescence.
A solution of luminol demonstrating chemiluminescence. L-R: A squid and a firefly demonstrating bioluminescence.
L-R: A squid and a firefly demonstrating bioluminescence. A selection of electroluminescent blue LEDs.
A selection of electroluminescent blue LEDs. A radioluminescent tritium light source.
A radioluminescent tritium light source.