High resolution (250m per pixel) imagery from NASA’s MODIS satellite.
The ash plume is even more visible in the false-colour image:
High resolution (250m per pixel) imagery from NASA’s MODIS satellite.
The ash plume is even more visible in the false-colour image:
For a long time there have been reports of “earthquake lights”, aurora-like lights or colours that appear in the sky during, or just before, earthquakes. Their existence is not widely accepted, but a group of US and Russian researchers claim to have discovered a different earthquake-related atmospheric effect.
After the March 11 quake in Japan the researchers looked at atmospheric data from the days before the quake struck, and their data appears to show a noticeable heating effect in the atmosphere beginning three days before the quake.*
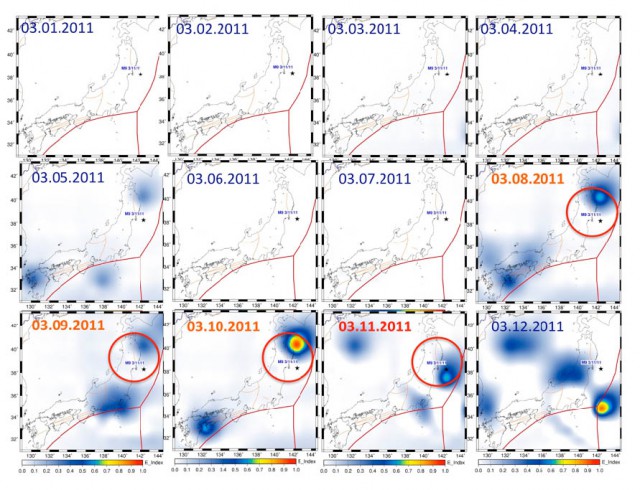 Red lines indicate tectonic fault lines and the star indicates the location of the March 11 earthquake.
Red lines indicate tectonic fault lines and the star indicates the location of the March 11 earthquake.
The article claims that this heating is due to a complex mechanism called the lithosphere-atmosphere-ionosphere coupling mechanism, in which the lithosphere (the rocky crust of the Earth), the atmosphere (the layers of gas surrounding the Earth) and the ionosphere (a layer of the atmosphere that contains particles that have been ionised by the Sun, where aurora occur) interact with each other.
They posit that small movements of the crust that occurred before the earthquake released radioactive radon gas (created by the radioactive decay of uranium and thorium in the crust) that was trapped there. This radon gas (either Rn-220 or Rn-222) is a highly-ionising alpha emitter. The researchers propose that the radon creates ionised particles in the air that cause water molecules to condense out of their vapour state. This condensation process releases energy, causing the surrounding atmosphere to increase in temperature.
Dimitar Ouzounov et al. 2011. “Atmosphere-Ionosphere Response to the M9 Tohoku Earthquake Revealed by Joined Satellite and Ground Observations. Preliminary results” arXiv:geo-ph/1105.2841v1

In the run up to World War II Niels Bohr’s Institute in Copenhagen had become a refuge for Jewish physicists. The Jewish physicist James Franck and the anti-Nazi physicist Max von Laue, concerned for their gold Nobel Prize medals, both left them there for safekeeping. When Hitler’s army invaded Denmark, Bohr was worried that the invading German soldiers would steal the medals but his friend, the Hungarian physicist George de Hevesy, came up with an ingenious solution.
de Hevesy dissolved both medals in aqua regia, a highly corrosive 1:3 mixture of nitric acid and hydrochloric acid. This formed a transparent yellow liquid, chloroauric acid, that he hid in plain site, simply leaving the jar on a shelf in the Niels Bohr Institute.
When he returned in 1945, after World War II had ended, the jar was still there. The gold was recovered from solution and the medals were restruck by the Nobel Foundation and returned to their rightful owners.
The Namaqua chameleon (Chamaeleo namaquensis) is really good at physics, even though he probably doesn’t realise it.

During the morning, when the Namib Desert in which it lives is colder, the chameleon turns its skin black so that it absorbs sunlight more efficiently and heats up quickly.
They can even split their colouring along their spines, white on one side and black on the other; absorbing heat on the black side whilst not radiating it on the white side.
The clip is taken from the BBC David Attenborough series Life.
A vapour is created when a substance forms a gas at a temperature below its boiling point; the vapour pressure of a liquid is the pressure of this vapour. A liquid boils when the vapour pressure of the liquid is equal to the atmospheric pressure of the air above it.
You can therefore boil a liquid in two ways: by heating it so that the vapour pressure increases to match the atmospheric pressure; or by decreasing the atmospheric pressure until it matches the vapour pressure at whatever the ambient temperature is.
In this video you can see me boiling water at room temperature: a beaker full of water at room temperature is placed in a vacuum chamber and the pressure lowered until it boils – the pressure gauge is on the right of the picture.
Notice how I can put my finger in the water both before and after boiling without scalding myself. It’s said that it’s impossible to make a good cup of tea at the summit of Mount Everest because water boils at only 70.4°C and this isn’t hot enough to properly brew tea.