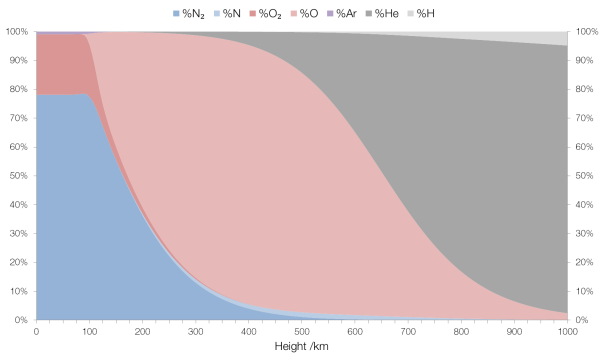
In researching a post about the Kármán Line I discovered the NASA MSIE E-90 atmosphere model (thanks to Rhett Allain) which models the composition of Earth’s atmosphere up to an elevation of 1000 km. I found it very interesting.
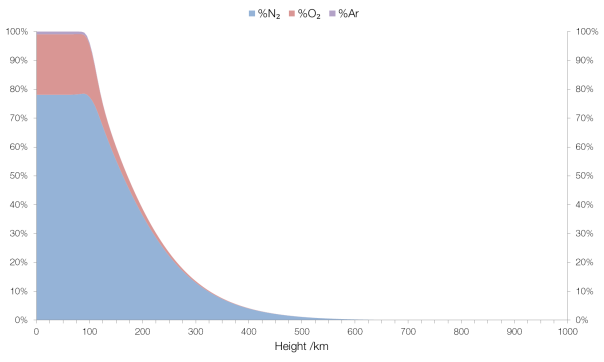
Up to around 100 km the composition is fairly “normal”, in that it’s what we surface-dwellers would expect: mostly molecular nitrogen (N2 rather than N) and molecular oxygen (O2) with a small amount (0.93%) of argon and traces of some other gases (carbon dioxide, neon, etc.).
After 100 km the percentage of molecular nitrogen and molecular oxygen decrease sharply, and there is a similarly sharp increase in monatomic and triatomic oxygen, better known as ozone (i.e. this represents the “ozone layer”). There is also a small increase in the percentage of monatomic nitrogen and nitrogen compounds, and argon disappears entirely.
By 200 km ozone dominates, and this continues to about 650 km where helium takes over as the predominant component. Monatomic nitrogen and nitrogen compound concentration peaks at around 500 km, with an overall concentration of 1.6%.
By the time we reach an elevation of 1000 km helium makes up 93% of the atmosphere. This is due to the fact that helium is an unreactive and very light atom (with a mass about one-eighth of oxygen) and thus isn’t held tightly by Earth’s gravitational field. (Helium is so light that it can escape Earth’s gravity entirely.) The bulk of the remainder is hydrogen, also prevalent due to its low mass (about one-sixteenth of oxygen’s).
The concentration of “normal” gases in the atmosphere with elevation.
The concentration of less common gases in the atmosphere with elevation.
It’s important to note that the graphs above all show concentration as a percentage of the total number of particles of gas in the atmosphere, rather than by mass or volume. The atmosphere becomes incredibly thin at high elevations, so that particles of gas may travel many kilometres between collisions, and if absolute concentrations were used instead, the graph would look very different (and be completely unusable, which is why I haven’t included it here).
I thought I’d give the NASA MSIE E-90 atmosphere model a try. I’m getting different results from yours. For instance, I’m getting an O and H peak at 100 km, which is almost what I would expect. I would expect H to peak at 120 km. The temperature data from the model also seems high. I was expecting 0 C and the model gave me 127 C at 120 km. I don’t see an option for charting O3.
Here’s the app I’m using. Perhaps you’re using a different one.
http://ccmc.gsfc.nasa.gov/modelweb/models/msis_vitmo.php
As I understand it, the upper heterosphere, at a distance greater than 100 km (the limit of Earth’s atmosphere, the Kármán line) and up to 120 km altitude, is composed almost exclusively of hydrogen. The temperature at 100 km is -86 C and the temperature at 120 km is 0 C. There are seasonal variation in molecular oxygen near 100 km altitude. The density of hydrogen (H2), is 0.55 ppmv within the heterosphere.
I’m rather new to this info. Can you tell if I’ve obtained old and out-dated data?
But, aside from all that, I actually stopped by with a question. I think you’ve answered it partially already – “particles of gas may travel many kilometres between collisions”. Given my current understanding of the homosphere and heterosphere, I’m wondering why there hasn’t been a notable reaction between the oxygen and hydrogen. Aren’t particle proximity and a spark all that are needed for an explosion?
You’re comment would be appreciated. Thanks for posting your graphs and naming your data source. Please do let me know if you have a different app link.
Thanks again. :-)
Theresa,
The date and (more so) the longitude/latitude will make a big difference to the results you achieve. I suspect that is the source of our differing results. I used the same source as you.
With regards to a reaction between hydrogen and oxygen, you need a certain concentration for those sides of the fire triangle (i.e. fuel and oxygen) to be complete.
Thank you for the answers, Mr. Reid. The numbers I’m getting are based on the app’s default coordinates.: lat=55, long=45. I’m using Jun 21 2014 for the date this time, but the defaults were used previously and there’s no noticeable difference. I’m outputting to plot. It seems that the plot doesn’t accurately represent the data in the list output. For example, in the plot, there are no reported N2, O2, H2, or Ar above 50 km.
At the Kármán line, O = 2,336,000 molecules / km3 and H = 121.3 molecules / km3, according to the data I see in the list output. At 3 kg of hydrogen per second escaping the troposphere, I suppose it’s just a matter of time for concentrations to accumulate.
Thank you for responding.
Here’s a thought – maybe the Tunguska Explosion of 1908 was a result of an Oxygen-Hydrogen collision. Monatomic oxygen is present from 80 km and up.
No, definitely not. There is really no evidence that the Tunguska Event was anything other than a meteor strike (though it wasn’t actually a “strike”, the meteor exploded in mid-air).
LOL! Everyone has an opinion on Tunguska. Actually, it’s official status as a meteorite is still ‘doubtful’ and its classification is ‘unknown’.
http://www.lpi.usra.edu/meteor/metbull.php?code=24082
Thanks again. :-)
I don’t think you understand how science works.
I like the way Theresa thinks that more time is required for “concentrations to accumulate.” As if the billions of years to date was not sufficient…
Hi Mr. Reid,
You have some excellent graphics on this website.
However, I believe you are confused or made a mistake. There is very little to no ozone at 100 km and it certainly doesn’t dominate up through 650 km. I believe you meant to say monatomic oxygen. Ozone (O3), would be quickly dissociated by x-rays, which are prominent above 100 km.
Also, the ‘ozone layer’, is a layer of ozone that exists in the lower stratosphere that peaks around 20 km and extends to around 40 km. Above that, there is a secondary ozone maximum that exists in the mesosphere (~70 km) in the absence of light, but there is very little to no ozone above that.
As your graphics show, monatomic ozone is the primary constituent above 100 km until helium overtakes it near the exosphere.
I have a question if I may. Why doesn’t the air composition (N2 Vs O2) change at low altitudes? I would expect the difference in weight (28 Vs 32) would play a role in reducing the percentage of O2 as you go up
Ww Rd, y ‘r ttl ss, lk mst scnc tchrs! (r y bld t?) thnk t s vry ‘nscntfc’ f y t sy “n dfntly nt” t n ctlly vry ntrstng hypthss n th Tngsk vnt. r y jst vry jls yr mnd dsn’t wrk t ll? Scnc s th nw rlgn rght, gtt spt tht dgm. m gld s yng ppl r rjctng th brnwshng nd ll th prpgnd fld scntsts/tchrs k ppl wh rn’t tht brght spw nwdys. dn’t thnk y ndrstnd hw ctl ntllgnc wrks.