A convincing argument can be made that the Atacama Desert in Chile and Argentina is the driest place on Earth. The average rainfall is one millimetre per year and some weather monitoring stations have never detected rain. This week eighty centimetres of snow fell in the region.
This is what the desert normally looks like:
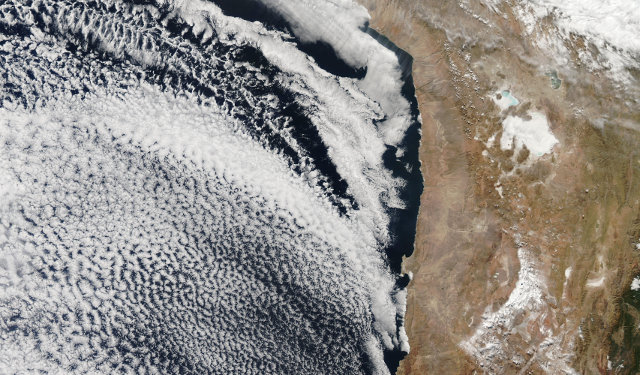
And this is how it looks now:
 The South Pacific is on the left of the image and Chile and Argentina on the right.
The South Pacific is on the left of the image and Chile and Argentina on the right.
It’s difficult to differentiate between cloud and snow in the true-colour image above, but the false-colour image below makes the difference more obvious.
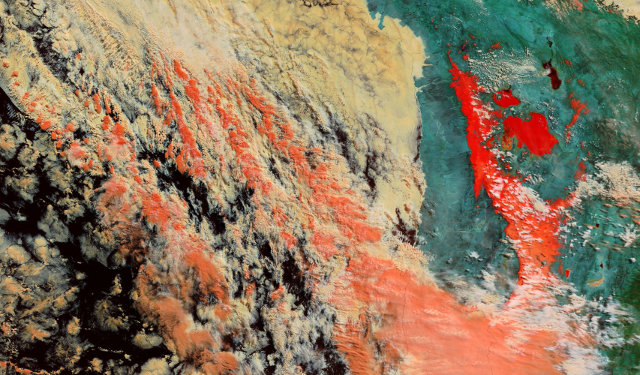
Areas covered in snow are bright white in the visible part of the spectrum and all the visible light detected by the camera in the second image is mapped to the red channel of the image. Ice is very absorbent in the shortwave infrared region that is mapped to the green and blue channels and therefore ice appears bright red.