For a long time there have been reports of “earthquake lights”, aurora-like lights or colours that appear in the sky during, or just before, earthquakes. Their existence is not widely accepted, but a group of US and Russian researchers claim to have discovered a different earthquake-related atmospheric effect.
After the March 11 quake in Japan the researchers looked at atmospheric data from the days before the quake struck, and their data appears to show a noticeable heating effect in the atmosphere beginning three days before the quake.*
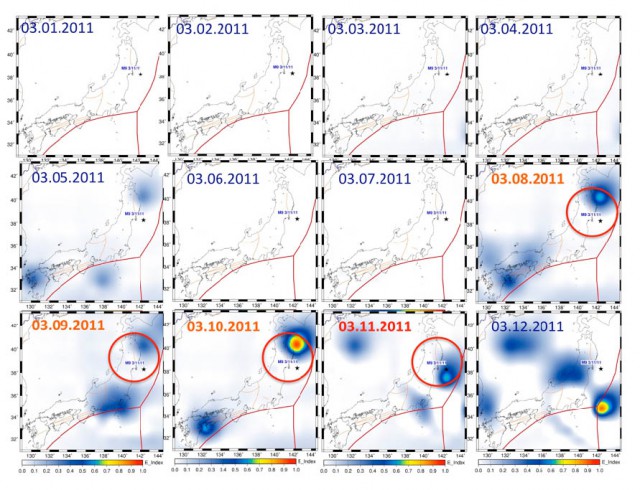 Red lines indicate tectonic fault lines and the star indicates the location of the March 11 earthquake.
Red lines indicate tectonic fault lines and the star indicates the location of the March 11 earthquake.
The article claims that this heating is due to a complex mechanism called the lithosphere-atmosphere-ionosphere coupling mechanism, in which the lithosphere (the rocky crust of the Earth), the atmosphere (the layers of gas surrounding the Earth) and the ionosphere (a layer of the atmosphere that contains particles that have been ionised by the Sun, where aurora occur) interact with each other.
They posit that small movements of the crust that occurred before the earthquake released radioactive radon gas (created by the radioactive decay of uranium and thorium in the crust) that was trapped there. This radon gas (either Rn-220 or Rn-222) is a highly-ionising alpha emitter. The researchers propose that the radon creates ionised particles in the air that cause water molecules to condense out of their vapour state. This condensation process releases energy, causing the surrounding atmosphere to increase in temperature.
Dimitar Ouzounov et al. 2011. “Atmosphere-Ionosphere Response to the M9 Tohoku Earthquake Revealed by Joined Satellite and Ground Observations. Preliminary results” arXiv:geo-ph/1105.2841v1


