I’ve recently been experimenting with making spherical ice cubes for cocktails.

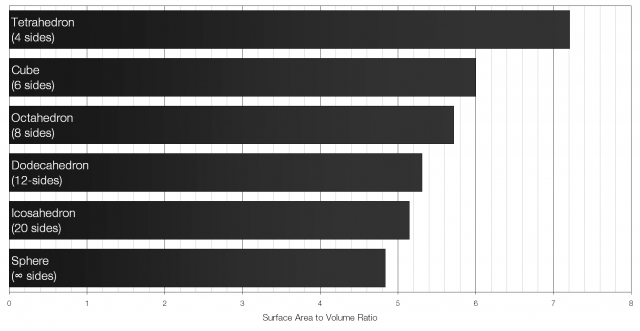
But why go to all the fuss of making spherical ice cubes? What’s wrong with regular ice cubes? The answer is surface area to volume ratio: the volume of the ice provides the cooling effect but the surface area controls how fast the ice melts – the lower the surface area to volume ratio the longer the ice will take to melt for the same cooling effect. Essentially, a lower surface area to volume ratio keeps your drink cold, but stops it from becoming too diluted.
A cube with sides of length x will have a volume of x3 and a surface area of 6x2. The surface area to volume ratio for a cube is therefore 6 to 1 (6:1). Of all the Platonic solids (solids with identical faces) the icosahedron has the lowest surface area to volume ratio.

Of all the regular shapes a sphere has the lowest possible surface area to volume ratio. That is what makes it particularly well suited for cooling drinks.
The production of spherical ice cubes is also quite interesting. They’re usually made in an extremely elaborate process using large blocks of ice that are then shaped using metal “presses” (usually made of copper or aluminium as they are very good conductors of heat).