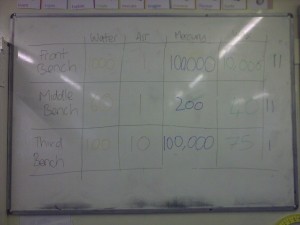
It turns out that estimating density is something that pupils find really hard.

I asked a class of pupils to estimate the density of four substances: air, water, mercury and wax. Before you read any further, try to estimate (in kilograms per metre cubed) the density of these four.
In case you can’t read the photograph, their guesses (in kg/m3) were as follows:
- Water – 1000, 60, 100
- Air – 1, 1, 10
- Mercury – 100000, 200, 100000
- Wax – 10000, 40, 75
And the correct values:
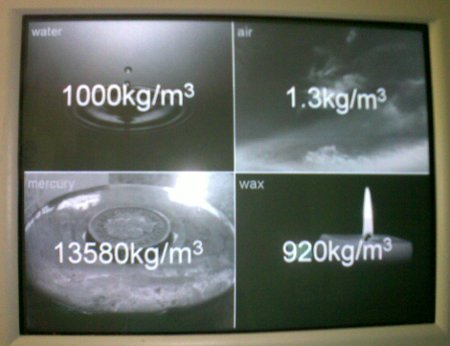
They were much closer to the correct answer for air than for anything else, but probably only because we’ve discussed it before when looking at mass. Interestingly, taking the geometric mean of the suggested values for mercury gives a value of 12600kg/m3; very close to the correct value of 13580kg/m3, but this is almost certainly a fluke.
So why is estimating density so difficult?
Pupils have far less trouble estimating mass, and less trouble estimating volume (though converting from cm3 to m3 is something they find very taxing). I think the problem comes from a lack of reference points – they’ve all held a kilogram in their hand, and have used metre rulers frequently so can envision a box 1m×1m×1m. The main point of this exercise is to give them reference points. For example: if they calculate the density of a material to be 1200kg/m3 then they know it should sink in water, and something with a density of 1kg/m3 should be lighter than air.