Some of the quantities measured in physics cover a very large range of values and this can make displaying measurements of their value difficult or confusing.
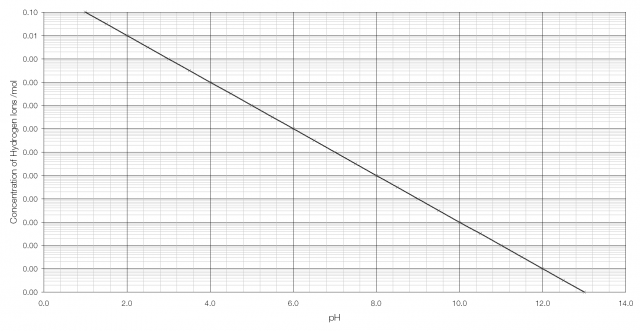
pH, traditionally thought of as a measurement of acidity, but actually a measurement of the concentration of hydrogen ions,* is one such quantity. Stomach acid has a concentration of hydrogen ions of 0.1 per mole; bleach has a concentration of hydrogen ions of 0.0000000000001 per mole.
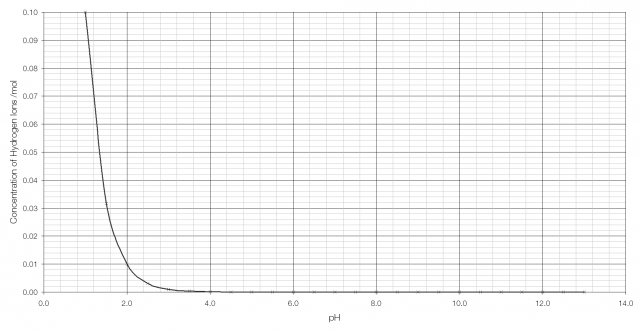 In order to have a sensible scale by which to judge acidity a logarithmic scale is used: pH is the negative of the logarithm of the concentration of hydrogen ions, so for stomach acid pH = −log(0.1) = 1.0 and for bleach pH = −log(0.0000000000001) = 13.
In order to have a sensible scale by which to judge acidity a logarithmic scale is used: pH is the negative of the logarithm of the concentration of hydrogen ions, so for stomach acid pH = −log(0.1) = 1.0 and for bleach pH = −log(0.0000000000001) = 13.
As can be seen from the graph above, it becomes very difficult to tell the difference between H+ concentration beyond pH 2 or 3. But on a logarithmic scale the difference is clearly visible:
There has been some fuss on various blogs about a chart from the Minnesota Dental Association (58kB, .PDF) listing the acidity of various sweets. One sweet, WarHeads Sour Spray is listed as having a pH of 1.6, which when compared with battery acid at pH 1.0 sounds very alarming. But when the logarithmic scale is taken into account an increase of 0.6 on the pH scale is equivalent to a four-fold decrease in acidity – Sour Spray is only one-quarter as acidic as battery acid (that’s still pretty acidic, by the way, and not terribly good for your teeth).
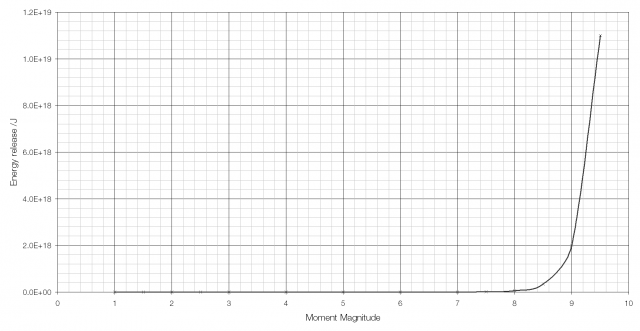
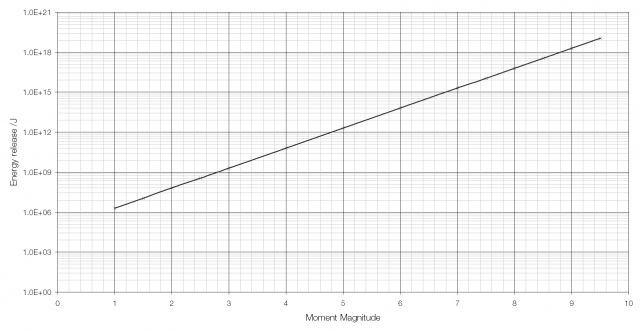
The moment magnitude scale used to measure the strength of earthquakes is another logarithmic scale. Earthquakes vary in size (i.e. in the energy they release) from the giant MW 9.5 earthquake in Chile in 1960 to tiny tremors caused by large vehicles going past and so a logarithmic scale is required. Because of the way that the moment magnitude scale is calculated an increase in moment magnitude of 1.0 indicates a 31.6-fold (101.5) increase in the amount of energy released (an increase in moment magnitude of two is equivalent to a 1000-fold (103) increase).
* For the sake of simplicity I’m ignoring the effect of the activity factor, the tendency of hydrogen ions to interact, on pH.