The temperature of a substance is a measure of the average kinetic energy of the particles in that substance. As the average kinetic energy of the particles increases (i.e. they move faster), the temperature of that substance increases.
Some of the particles in a very hot substance will be moving slower than some of the particles in a very cold substance, but the average speed of the particles in a hot substance will be faster than the average speed of the particles in a cold substance. The number of particles at each speed in a gas is governed by something called a Maxwell-Boltzmann distribution, and is shown for air in the graph below:
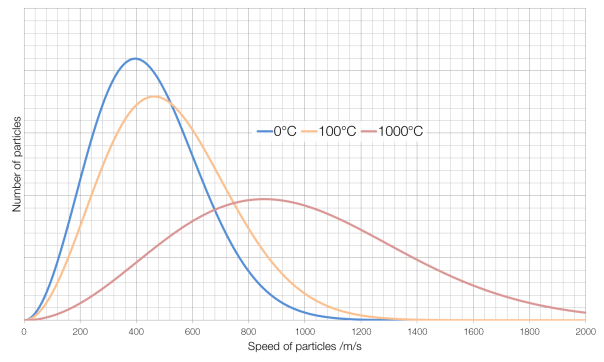
The average speed for particles of air* at 0°C is around 400 metres per second, for air at 100°C it is about 460?m/s and for air at 1000°C it is about 860?m/s. (Note also that at 1000°C there is far more variation in the speeds of particles than for air at 0°C.) At absolute zero, the coldest possible temperature, particles have a minimum of kinetic energy, and therefore the lowest possible speed. (They cannot have a kinetic energy of zero and actually be stationary because of something called degeneracy pressure.)
So why can’t we cool something all the way to absolute zero?
For something to cool down, it has to lose thermal energy. In order to lose thermal energy, this thermal energy has to go somewhere, and thermal energy only ever moves from hot to cold.** For example: a warm can of drink placed into a cold fridge loses thermal energy to its surroundings until it reaches the same temperature as the fridge’s interior.
Therefore, in order to bring something to absolute zero it would have to be surrounded by something that is colder than absolute zero, and this is impossible: hence you cannot achieve a temperature of absolute zero.
The closest we’ve ever got to absolute zero is less than 100 picokelvin, or 100 trillionths of a degree above absolute zero at the Low Temperature Lab at the University of Aalto in Finland. Interestingly though, it would be possible for something to feel colder than absolute zero due to wind chill.
* Obviously air is made up of different gases travelling at different speeds, so this is an average, weighted by the masses and prevalences of the different gases that make up air.
** It would actually be more accurate to say that the net movement of thermal energy is always from hot to cold. Some energy may go from cold to hot, but more will always go in the opposite “direction”.
Thank you for this super clear write up! I was reading about absolute zero in an Asimov book and was wondering why we can’t reach it…
” In order to bring something to absolute zero it would have to be surrounded by something that is colder than absolute zero.” So that statement is also true for any other lower temperature. So according to it, to attain any lower temperature we have to use a temperature that is lower than the desired temperature?? How could we do that??
Isn’t there any other way to attain the lower temperature??
By applying external work we can do that.
E.g. In refrigerators we transfer heat from cold reservoir to hot reservoir by applying electrical work.
Okay, well you use a refrigerator to reach absolute zero and let me know how you get on.