When people think of nuclear fuel they tend to think of uranium and plutnonium, or more specifically their fissile isotopes: uranium-235, plutonium-239 and plutonium-241. But there is another fissile isotope that doesn’t get the attention it deserves: uranium-233.
A fissile isotope is one that can sustain a nuclear chain reaction. There is only one naturally-occurring fissile isotope: U-235 which makes up 0.7% of mined uranium (the other 99.3% being non-fissile U-238). Plutonium-239 and -241 are both “bred”, created artificially in a reactor: Pu-239 from the inert U-238 and Pu-241 from Pu-240 which is itself bred from Pu-239.
Making plutonium-239:
In the first stage U-238 is bombarded with neutrons (n) to create U-239. This U-239 then undergoes beta decay* to form neptunium-239:
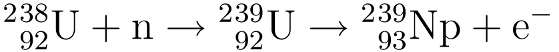
This neptunium then undergoes a second beta decay to form Pu-239:
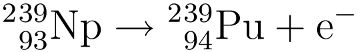
Making plutonium-241:
To create plutonium-241 the plutonium-239 from the previous step is bombarded with neutrons to form first Pu-240 and then Pu-241.
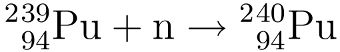
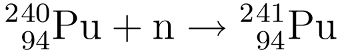
Breeding uranium-233 from thorium:
Uranium-233 is produced by bombarding thorium-232 with neutrons to create Th-233 which then undergoes two beta decays to form U-233. This can all be done inside the reactor itself.
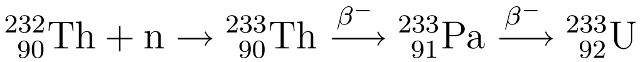
Using thorium as a nuclear fuel has a number of significant advantages: it is made up of only one isotope which means that no costly (in both financial and energy terms) enrichment processes are necessary and thorium is at least four to five times more abundant in Earth’s crust than uranium.
Thorium can be used in a molten salt reactor, where it is dissolved into uranium fluoride to form a fluid that is both fuel and coolant (the full name of this reactor is the liquid fluoride thorium reactor). The advantage of using molten thorium as both fuel and coolant is that the reactor then has passive (“fail-safe”) safety: if the fuel begins to overheat then the reaction rate decreases, making a meltdown impossible.
The reaction takes place at a pressure of one atmosphere, meaning no pressure containment vessels are needed. Thorium reactors produce far less waste than uranium reactors do, and the waste produced is far safer: after 10 years 83% of the waste can be sold to recyclers and reused.
More information:
- Uranium Is So Last Century — Enter Thorium, the New Green Nuke by Richard Martin in WIRED magazine.
- Energy from Thorium by Kirk Sorenson
- Thorium from World Nuclear Association
* Completists will notice that I’ve missed out the electron antineutrinos produced in beta decay; I’ve removed them for simplicity since they don’t really play a role here.
It can be used to make a Bomb, too. In fact, the U.S. detonated one Bomb
with a Uranium 233 core; it had a yield of 22 kilotons, about the same as
the Bomb used on Nagasaki. Plutonium would have given a higher yield
(33 kilotons) but 22 kilotons is still enough to trash the downtown section
of a medium sized city and kill tens to hundreds of thousands of people.
I assume you’re talking about Operation Teapot. That test used a composite U-233 and plutonium pit. Your assertion that it had “a Uranium 233 core” is incorrect.